The AK-antiVEGF-101 Clinical Trial is for individuals affected by unilateral vestibular schwannoma. The purpose of this trial is to evaluate whether the study drug, administered once using the study device, is safe at different dose levels. Researchers in this trial will also evaluate whether the study drug is able to stabilize or improve hearing and/or shrink the size of the tumor in individuals affected by vestibular schwannoma. This trial is sponsored by Akouos, Inc., a wholly owned subsidiary of Eli Lilly and Company.
You may be able to join the trial if the following apply to you:

You are at least 18 years old.
You have significant hearing loss in one ear caused by vestibular schwannoma.

You have had an MRI scan of the head in the past two years.
You have not received surgery or radiation to treat the vestibular schwannoma.

You do not have vestibular schwannoma on both sides.
Other eligibility criteria will also apply.
Trial participants can expect the following:
Participation in a clinical trial is voluntary. You can ask any questions you have and may leave the trial at any time, for any reason.
The study drug is a type of gene therapy. It is designed to deliver a sequence of DNA to the cells of the inner ear. This sequence may enable the cells of your inner ear to produce and then release a protein referred to as anti-VEGF into the fluid of your inner ear. The anti-VEGF protein may then travel to the vestibular schwannoma, where it may block a growth factor called VEGF that is made by the vestibular schwannoma. By blocking VEGF, the study drug may help to slow or reverse the growth of the vestibular schwannoma and may also help to improve or stabilize your hearing.
The study drug is administered to the inner ear during a surgical procedure, using the study device.
Watch a short video provided by the American Society of Gene & Cell Therapy to learn more about gene therapy.
The study device is used to help deliver the study drug to the inner ear.
Watch a short video to learn more about the administration procedure.
Trial participants who meet all eligibility criteria will receive one dose of the study drug. There is no placebo in this trial.
Both the study drug and the study device are investigational. This means they can only be used in clinical trials, such as AK-antiVEGF-101, and have not been approved for sale by any regulatory authorities, such as the U.S. FDA. This is the first time the study drug is being used in humans. The study device has been used in another trial (unrelated to vestibular schwannoma), which is currently ongoing.
A vestibular schwannoma is a non-cancerous tumor. It forms along the nerve that runs from the inner ear to the brain. A vestibular schwannoma can affect the body's ability to send nerve signals from the ear to the brain. This may lead to symptoms such as hearing loss and balance issues.
Acoustic Neuroma Association: https://www.anausa.org/
Here are some common questions and answers about trial participation.
Clinical trials help scientists and doctors explore whether a medical strategy, drug, and/or device is safe and effective for people. Before a new study drug and/or study device can be approved for sale and made available to the public, it must go through several phases of clinical research. Each phase helps researchers, regulators, and patients learn more about the medical strategy, drug, and/or device. Clinical research relies on volunteer participants.
Watch this video to learn more about clinical trials.
To enroll in a clinical trial, you must first sign an Informed Consent Form (ICF). The ICF contains information about the trial, including trial goals, how long the trial will last, risks related to participation, the tests and procedures that will occur, and participant responsibilities if you decide to participate in the trial.
Watch this video to learn more about ICFs.
Trial participation involves visiting a trial site regularly, receiving a study drug, and having assessments to monitor your health. You can still see your regular doctor (and other healthcare providers), and you should let them know that you are participating in a gene therapy clinical trial.
Participation in a clinical trial is your choice, and you may stop at any time. You may be eligible for travel coverage or reimbursement for expenses.
Watch this video to learn more about what to expect once enrolled into a trial.
If you have additional questions about participating in this trial, contact a trial site near you, or email us at AkouosClinicalTrials@Lilly.com.
Contact the trial site to learn more.
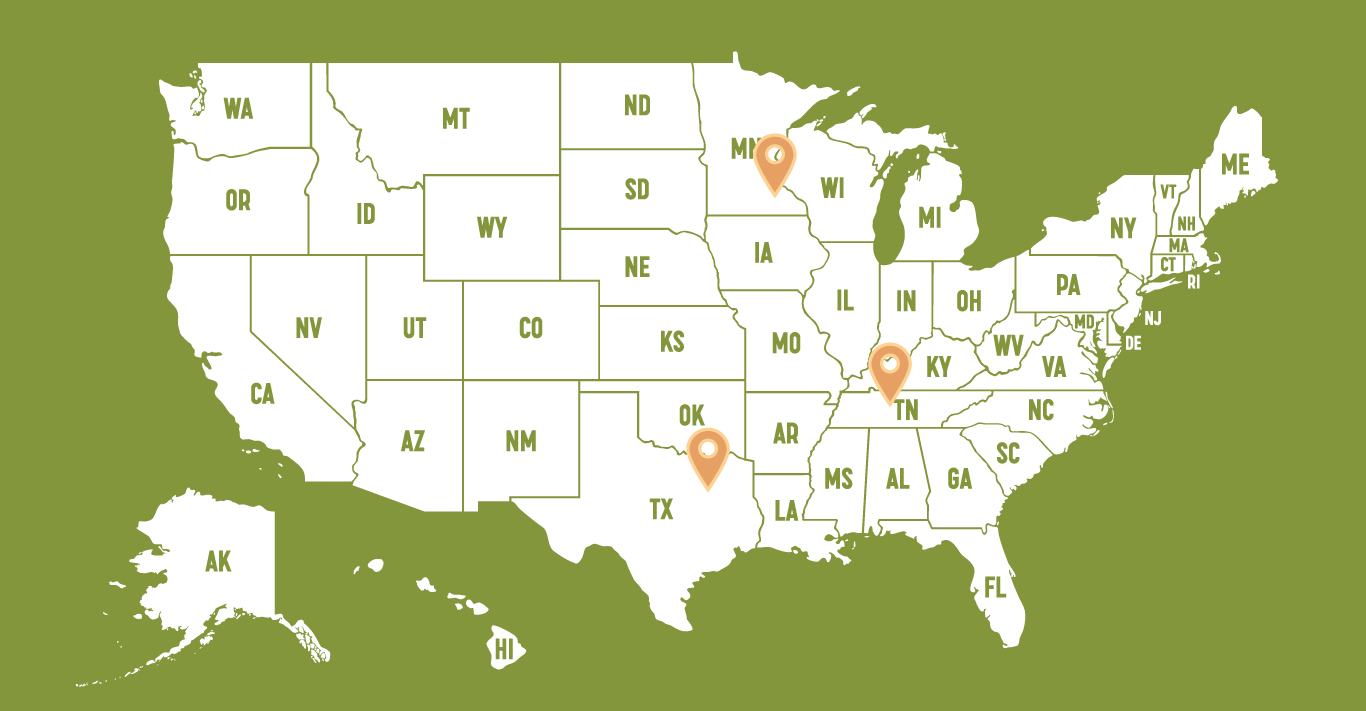
Mayo Clinic - Rochester
University of Texas Southwestern
Vanderbilt University Medical Center
You may be eligible for travel coverage or reimbursement. For more information, please contact us at AkouosClinicalTrials@Lilly.com.